When you envision FIS, you will see a reliable, long-term partner with an integrated contract manufacturing organization able to manage small-molecule APIs and Intermediates projects from Development to Commercial production.
With our quality management system, R&D capabilities, and production technology platform, FIS represents your partner of choice for building a solid business relationship.
CDMO
Contract Development
CDMO
Contract Manufacturing
GX
Generic API
Development & Manufacturing
CDMO VET
Contract Develop. & Manufact.
for Veterinary business
CDMO S
Services for Sterilization, Micronization, Lyophilization, Spray Drying

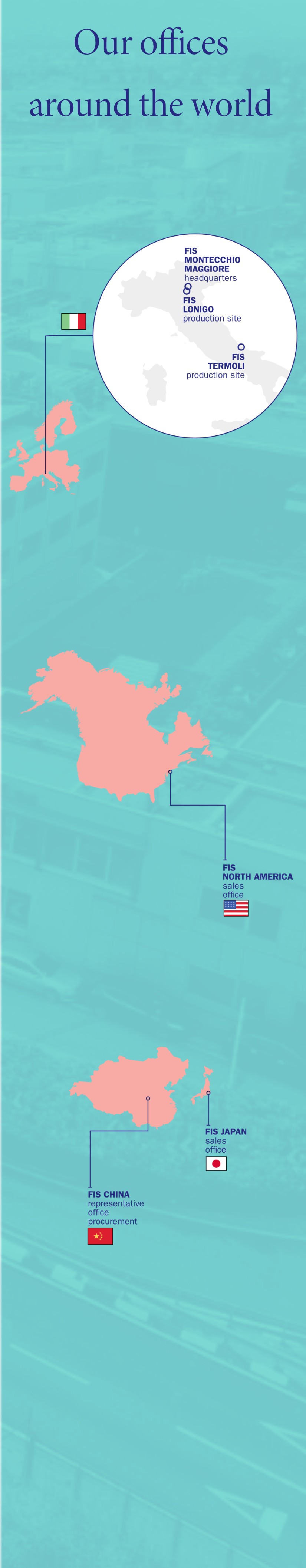
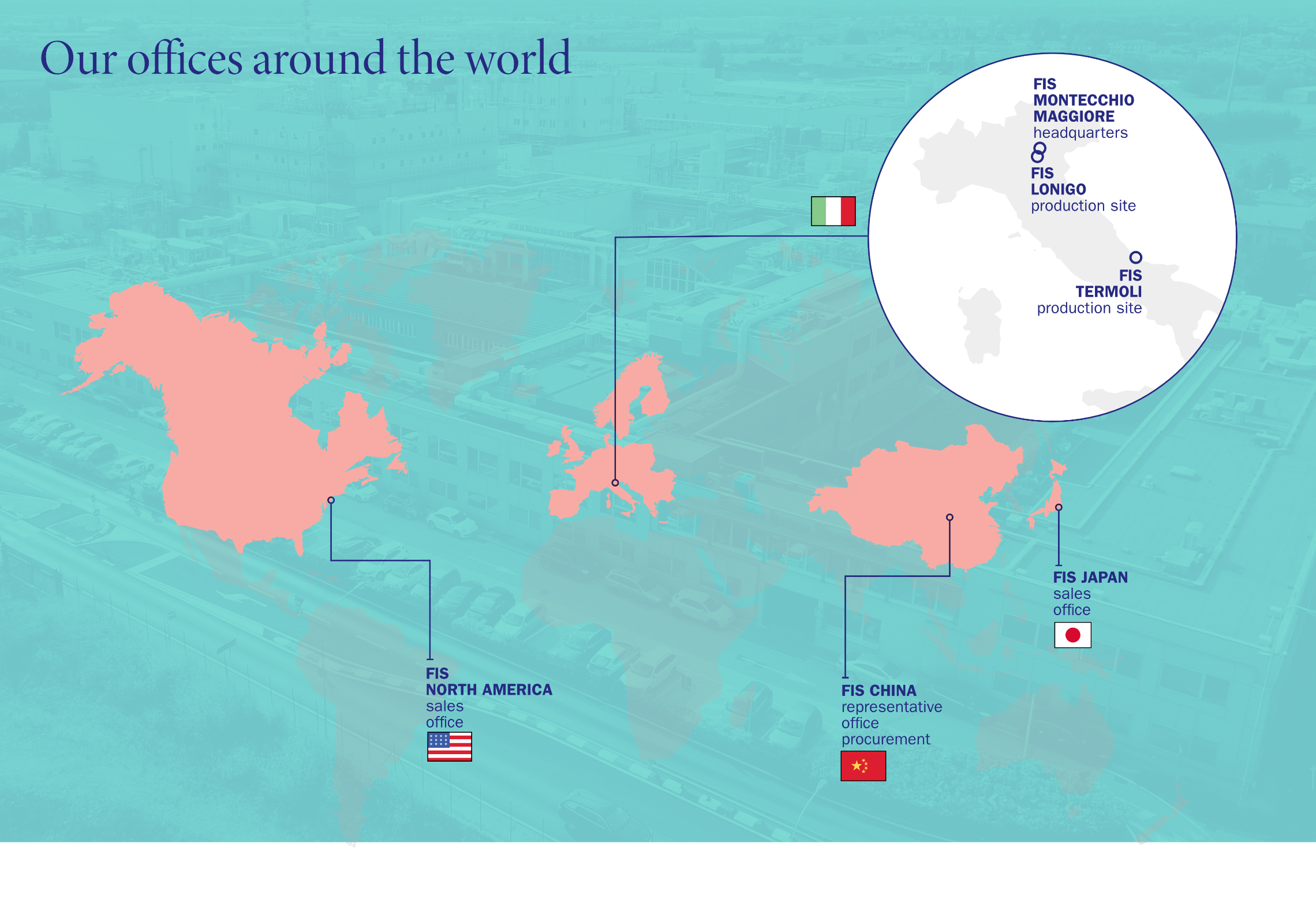
FIS is authorized by the Italian Medicines Agency (AIFA) and the Italian Ministry of Health to produce more than 100 APIs of various therapeutic classes, for anticancer treatments, steroids, psychotropics and non-beta lactam antibiotics, as well as APIs for veterinary use. Production takes place in dedicated or segregated plants or on multi-purpose lines, depending on product category. FIS’ Montecchio facility was also authorized in 2016 to produce semi-finished pharmaceutical product batches with Spray Dry technology.


Our excellence and growth go hand in hand with those of our employees; people who are encouraged to express their full potential across all roles and responsibilities. Today, we are a company of over 1,750 highly specialized personnel carrying out innovative and challenging projects every day: 250 involved in research and development, 420 graduates, 320 scientists, 70 engineers, of which 50 chemical engineers.